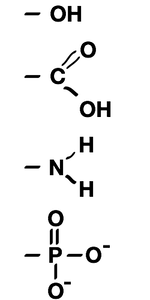
Biochemistry Labs
Biochemistry labs & metabolism lesson plans designed for high school biology and middle school life science teachers. A great biochemistry lab involves dehydration synthesis that lets students model paper molecules and form water. Metabolism worksheets about monomers and polymers are included. Click the Free Lesson Plan (PDF) link below or become a member to get access to the answer key and editable file. Free biochemsitry curriculum includes:
-
Biochemistry Lab - Metabolism & Food Labels  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Lab
Students will analyze their lunch based on the type of biomolecules in the food they would eat. This is a great metabolism lab that lets students relate to the metabolism, biomolecules, monomers and polymers.NGSS Standard
HS-LS1-6
MS-LS1-7 (Metabolism)
Published by NGSS Life Science
-

Metabolism & Monomer - Polymer Test Question Bank  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Test
Metabolism and Monomer Polymer question bank to help you build assessments. An active membership is required to view questions.NGSS Standard
HS-LS1-6
MS-LS1-7 (Metabolism)
Published by NGSS Life Science
-

What is Food Worksheet  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Worksheet
Students discover using their peer and class discussion what makes food and what is food mostly made out of. Good intro to biomolecules and metabolism.NGSS Standard
HS-LS1-6
Published by NGSS Life Science
-

Conservation of Matter Lecture Notes  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (PPTX) & Answer Key with Membership
High School Lecture
This PowerPoint introduces students to balancing a chemical reaction using photosynthesis as an example.NGSS Standard
HS-LS1-5
HS-LS1-6
Published by NGSS Life Science
-

Monomer Polymer Worksheet - Vocabulary  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Worksheet
This is a good practice worksheet for students to practice matching monomers with their polymers. Simple and effective.NGSS Standard
HS-LS1-6
Published by NGSS Life Science
-

Metabolism Worksheet  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Worksheet
In this metabolism worksheet, students match metabolism vocabulary to the types of food they eat. The concepts of dehydration synthesis and hydrolysis are also included.NGSS Standard
HS-LS1-6
MS-LS1-7 (Metabolism)
Published by NGSS Life Science
-

Biomolecule Quiz  Free Lesson Plan (PDF)
Free Lesson Plan (PDF)
Lesson Plan (DOCX) & Answer Key with Membership
High School Test
Assess student’s knowledge of monomers and polymers of macromolecules.NGSS Standard
HS-LS1-6
Published by NGSS Life Science